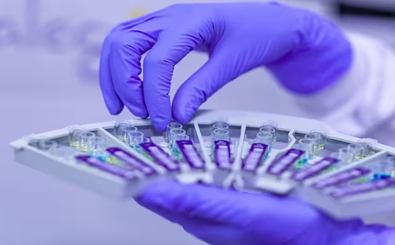
Using molecular diagnostics to improve diagnosis of TB & COVID-19 at point of care
Problem
The absence of specialized testing facilities and skilled technicians to diagnose TB results in several cases of TB going undiagnosed (“the missing millions”) – hampering efforts to eliminate the disease around the world. TB patients in low resource settings lack access to accurate and rapid TB diagnostic tests at point of care leading to delay in diagnosis and treatment initiation.
Solution
The Truelab Real Time Quantitative micro PCR system is a compact, battery-operated system which provides test results at the point of care within 90 minutes. This enables same day reporting and initiation of evidence-based treatment of tuberculosis, which reduces the risk of infection spreading while waiting for test results and facilitates faster recovery due to early initiation of treatment. It is significantly cheaper per test than other RTPCR tests. Truelab is a platform which supports tests for several diseases, including Covid19, making it a cost effective and capital efficient solution.
IMPACT
- IHF-supported deployment of Truenat in two districts in Uttar Pradesh demonstrated significant improvement in testing presumptive TB patients – with 92% of the samples tested reporting results on the same day. Treatment initiation time improved by 45% as compared to the baseline, and 96% patients initiated their treatment within seven days of test results. Truenat was also validated for effectiveness as a TB/COVID-19 diagnostic test at community health centres in Uttar Pradesh with support from India Health Fund.
- IHF with The Foundation for Innovative New Diagnostics (FIND) and Municipal Corporation of Greater Mumbai (MCGM) successfully implemented India’s largest bi-directional testing for TB and COVID-19 using Truenat in five high volume COVID-19 tertiary hospitals. The initiative screened more than 17,000 antigen negative symptomatic patients, and 13,500 COVID-19 OPD walk-ins went through the TB questionnaire followed by diagnosis using Truenat. Of these, 2.7% (379) were identified as TB presumptive and 78% (297) were tested for TB (until September 2021).
- Through a global pricing agreement signed in March 2023, Truenat has been approved for worldwide scale-up by the Global Fund, StopTB Partnership and USAID. The agreement will enable countries to procure the WHO endorsed-Truenat at internationally negotiated prices, making the technology more accessible and affordable across the world.
“The strong engagement with the user community as well as with public health experts that the India Health Fund enables, greatly smoothens the lab-to-market journey and facilitates deployment at scale. Such support from IHF has been invaluable for Molbio during the scaleup of the Truenat platform.”