1. About India Health Fund (IHF)
India Health Fund (IHF) is a not-for-profit organization that was set up as a collaborative initiative with Tata Trusts and The Global Fund to fight AIDS, Tuberculosis and Malaria. Our mission is to help reduce preventable deaths from communicable diseases and other public health risks. We do this by de-risking promising technology that have a potential to make a significant difference in the diagnosis, treatment and prevention of these diseases We collaborate with public and private sectors to facilitate the development, adoption and scale of these solutions. Our aim is to improve health outcomes, with a focus on primary care and in low resource settings.
India Health Fund (IHF) is a not-for-profit organization set-up as a section 8 company to support promising start-ups that aim to control infectious diseases through innovations in medical devices and digital health. IHF is a company established for charitable purposes, registered under section 8 of Companies Act 2013. IHF is also registered under section 12AB of Income Tax Act 1961.
IHF focuses on supporting technologies that control infectious diseases. A typical portfolio of IHF has platform technologies that cover screening, diagnosis, infection control, care-pathways, digital interventions, and surveillance of infectious diseases
India Health Fund (IHF) is a not-for-profit organization set-up as a section 8 company to support promising start-ups that aim IHF initially started as a programme of the Tata Trusts. Considering the long-term nature of the programme and its future expansion, Tata Trusts seeded and IHF was established as a separate legal entity in 2017 to manage the entire activities of IHF. Therefore, IHF’s principles and policies are aligned with those of Tata Trusts. Tata Trusts are currently the major funders of IHF.to control infectious diseases through innovations in medical devices and digital health. IHF is a company established for charitable purposes, registered under section 8 of Companies Act 2013. IHF is also registered under section 12AB of Income Tax Act 1961.
IHF is registered with Ministry of Corporate Affairs for undertaking CSR activities with registration number CSR00008848.
2. Funding thesis of IHF
No, IHF’s support consists exclusively of grant capital support to health innovations.
IHF supports innovations that are at TRL 4 stage and above. (Please refer Sr. No. 2 under terms used in the start-up ecosystem header).
Improving access to healthcare, reducing healthcare costs, promoting innovation & new technologies, improving health outcomes by providing quality services & addressing India specific challenges are few of the important reasons to incubate solutions in healthcare in India.
3. Portfolio Management at IHF
On an average a typical lifecycle of incubation varies between 18-24 months. However, it may be decided on a case-to-case basis as each portfolio needs vary.
Yes, at present two of IHF’s portfolio companies have entered the market which are Truenat (PCR platform for accurate & low-cost diagnosis of TB, Covid19 and 40 other conditions at point of care) & Qure.ai (AI-based platform to improve screening & detection of TB, Covid19 & other lung diseases at primary health care level).
Yes, IHF does play active engagement with its portfolios in following areas –
1. Milestone based funding support.
2. Need-based mentoring by a panel of experts for guidance on study design and validation, deployment, regulatory landscape, patent law, market analyses, and current policies.
3.Collective engagement with public/private stakeholders and access to the ecosystem of infectious diseases.
4. Access to resources for adoption in the health system and accelerated market adoption including support towards enabling demonstration through pilots, and advisory on go-to-market strategy.
4. Screening and evaluation process at IHF
Based on the proposal received at IHF, we follow the given process below to evaluate a funding request –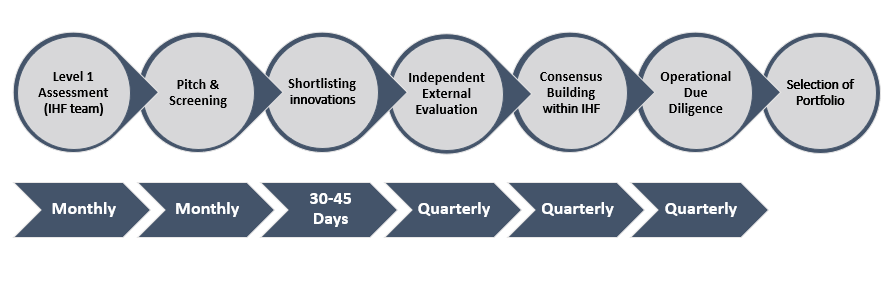
- Level 1 assessment: Internal assessment of technical proposals submitted to IHF based on multiple evaluation criteria in order to funnel out a relevant pipeline of innovations for further analysis.
- Inviting applicants for a comprehensive presentation of their innovation and start-up to the technical team at IHF.
- Internal consensus building and shortlisting promising innovations from the pipeline to be considered for support and external evaluations. Intimation to applicants to submit further information and relevant supporting documents on the detailed proposal.
- Virtual presentation to independent expert panel.
- Due diligence at operational and technical levels and a third-party financial audit of the awardee. Satisfactory completion of the same is mandatory for selection of a portfolio.
IHF sources innovations through following channels –
- Partner network of incubators, academic institutions, not-for-profit organizations and government agencies
- Direct entry through website and e-mails
- Call for proposals (with partners)
(Screening process is as per the diagram in the previous answer)
IHF has screening 1200+ innovations and has an active portfolio of 13 as of date.
Following are the eligibility criteria IHF follows –
- The innovation should have completed the proof-of-concept stage and validation stage
- Validation data should be readily available for justifying support for next stage
- Innovation must have a strong value proposition to be used in primary level of public health settings for control of infectious diseases
- The innovators must be registered and incorporated entities
- The innovation should be superior to the currently available solutions or approaches in terms of sensitivity, specificity, accuracy, safety, turnaround time, usability in peripheral settings, unit pricing
- The innovation should have the potential for scale-up across diverse settings
- It is desirable if the innovation is amenable to being multi-disease applicable
Following are the parameters IHF considers for the evaluation –
- Uniqueness of innovation
- Social Impact
- Stage of Development/ Validation data
- Problem Solution Fit
- User friendliness
- Scalability and Adoption potential
- Financial & Business Sustainability
- Entrepreneur/ Team Strength
- Platform/Multi-Disease potential
- Competitive edge
- Regulatory & legal readiness
IHF has a robust finance and accounting system to ensure financial prudence and also to keep detailed record of expenditure incurred. The system enables systematic tracking of utilization of the funds under approved expense heads and the corresponding budgets
5. Impact assessment at IHF
IHF has an extensive selection, onboarding and project review process to ensure social impact. The innovations need to be evaluated as per following criteria:
- Evaluation of each innovation based on tangible indicators of impact in public healthcare at the primary level.
- Identifying ground level problems and needs with help of different external experts to support the development and scale of innovations that addresses the critical problem.
- Support to start-ups with a common goal and commitment to address health issues of the underserved population and have a prototype of innovation ready.
- Selection of platform technologies that aid in control of various health issues of the under privileged and are sustainable in different geographies.
Evaluation of each innovation for outcomes and impact is conducted as per the following broad criteria:
- Is there a specific target group or issue for the social impact in the infectious disease space? What is it?
- Is there a clear understanding of the start-up of the targeted social issue?
- Are the metrics identified to track social impact? What are they? (Metrics to be charted as per each project with IHF and the start-up)
- Is the Impact sustainable? Is the Impact meaningful? How will you quantify and track that?
- How does the community benefit from the business or its services? What would happen in the absence of the company’s products and services?
IHF exercises a strict price control during the selection process for innovations and ensures the cost-effectiveness of the final product from the lab to last mile. Through formal agreements, the service provider commits to cause saving in public expenditure by providing the technology/product to the public institutions at an economical pricing. The service provider needs to be willing to discuss and determine the price point for the public good, being supported by IHF with relevant government departments or programmes for wider adoption at reasonable cost for public benefit.
Post the product incubation in lab, the innovation needs clinical validation, feasibility studies, Pilot Introduction, adoption by public healthcare, scale-up within different districts and states, pathways to global market access, business sustainability of start-ups through industry connects.
6. Collaborations with the government
Yes, IHF partners with various programs of Ministry of Health (National Vector Borne Disease Control Program, National TB Elimination Program), Municipal corporations, State and National Health Missions, ICMR, etc. For expert evaluations, project reviews, product development, clinical validations, access to test samples and sites, feasibility studies and pilot testing.
ICMR supports IHF for building pipeline and onboarding portfolios by providing critical evaluations of the innovations and also review existing portfolios for monitoring the path to disease control. ICMR also supports IHF’s portfolios for third-party clinical validations, feasibility studies and pilot testing of innovations.
7. Terms used in the start-up ecosystem
Incubators are institutions that support entrepreneurs in developing their businesses, especially in initial stages. These are organizations geared towards speeding up the growth and success of start-ups and early-stage companies. Incubation is usually done by institutions which have experience in the business and technology world. Incubation support includes providing technological facilities and advice, initial growth funds, network and linkages, co-working spaces, lab facilities, mentoring and advisory support.
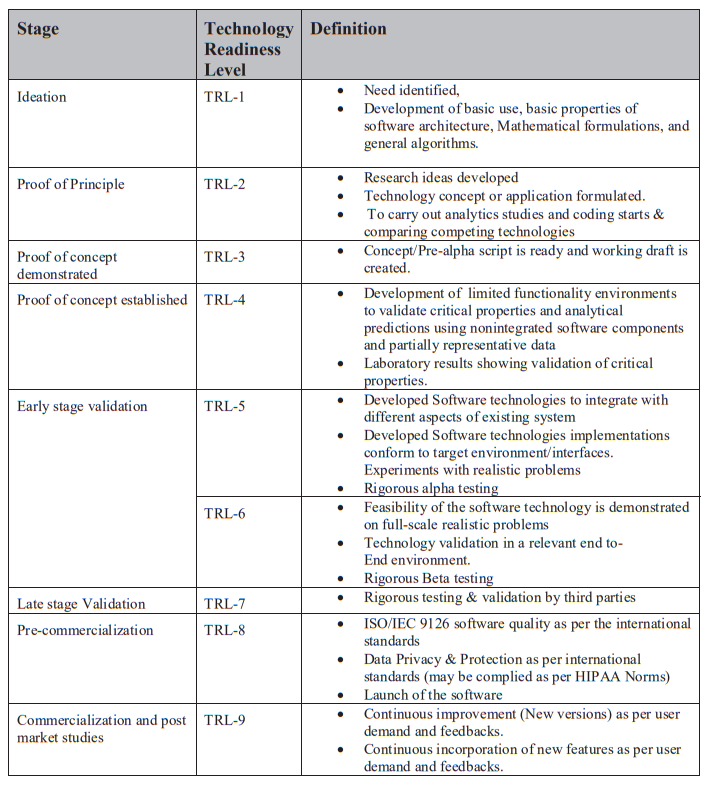
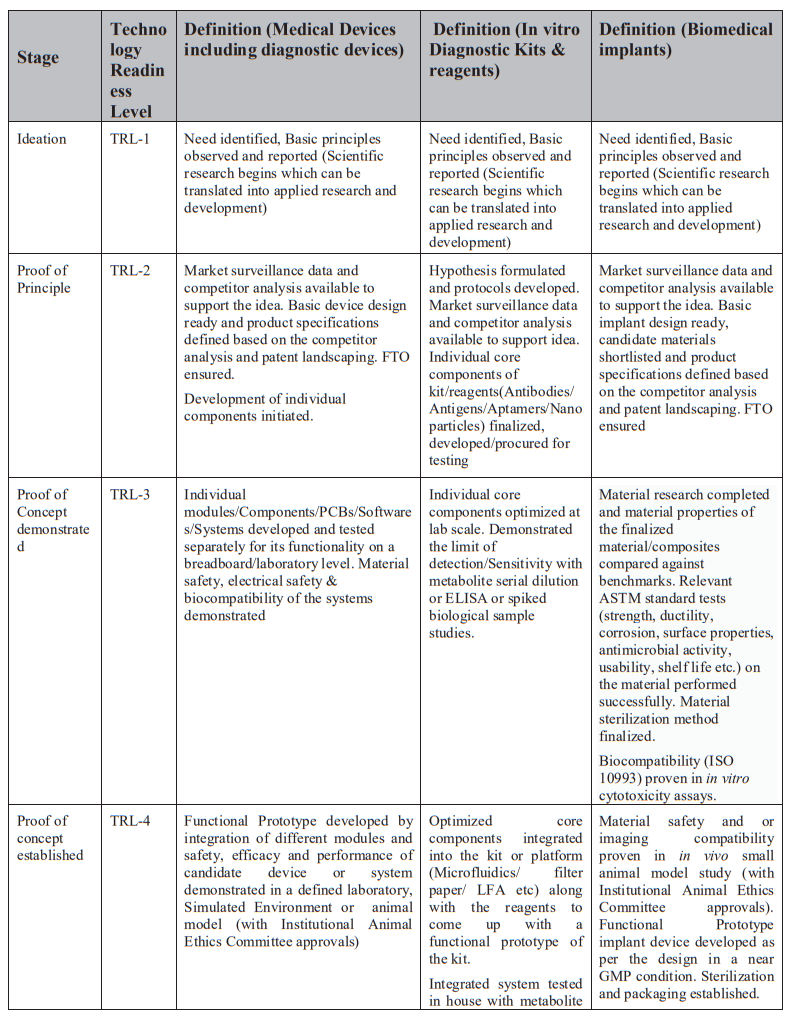
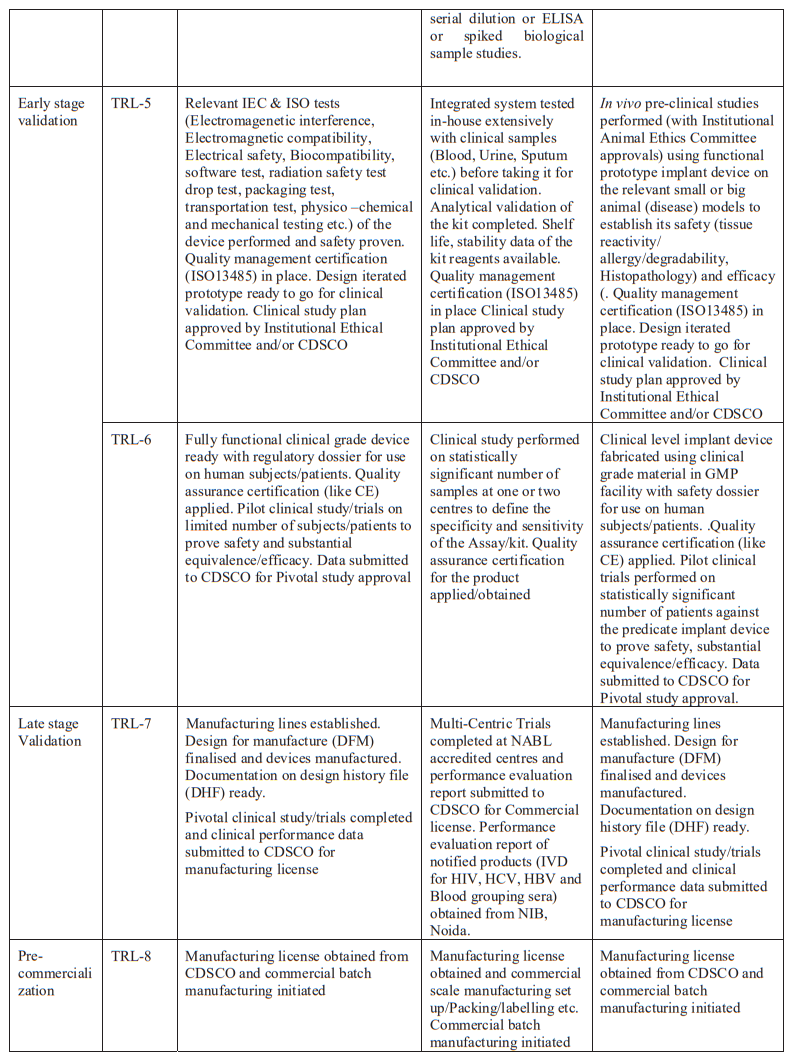
Technology Readiness Levels (TRL) are a type of measurement system used to assess the maturity level of a particular technology. Each technology is evaluated against the parameters technology level and is then assigned a TRL rating based on the how the technology progresses. There are nine technology readiness levels. TRL 1 is the lowest and TRL 9 is the highest.
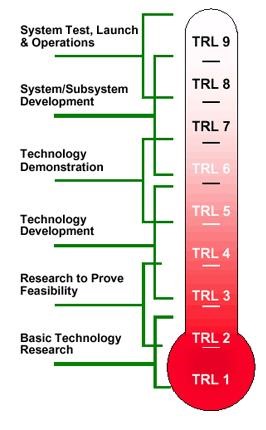
Refer Annexure 1 & 2 for further details on TRL
The Valley of Death is known as a metaphor for the lack of resources and expertise that impedes new ideas in their transition from lab to market. This gap also hinders innovation and adoption of new technologies
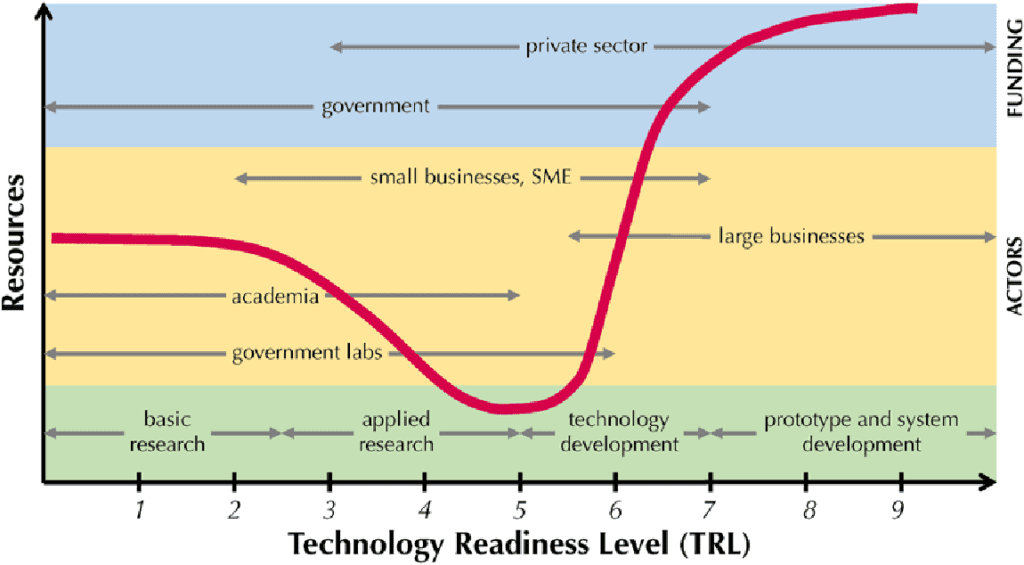
The above graph shows a dip in resources between TRL 3 and TRL 6 i.e., when the product is in the phase of applied research and technology development. Also, it is visible that there is a limited involvement and funding availability of government and private sector particularly in this phase of product development.
Adjacency refers to the transferability of innovations between Infectious diseases and other disease areas. While IHF has supported the development of diagnostic tools and technologies with a bias towards infectious diseases, the same platform technologies can be repurposed through support from other stakeholder partners towards use across other health domains including NCDs, MNCH etc.
Evidence generation is a process where one produces proof of the workability/effectiveness of a particular intervention on the target outcomes/impacts. Beyond simply proving if an intervention is effective and safe, it also involves proving that the new treatment addresses market gaps that the current products do not meet.
8. Overview of infectious diseases elimination
- Innovation Development – Need for patient capital
Infectious disease innovations need access to patient capital across the lifecycle of development (lab-to-market) as deep science and deep technology innovation need time for development, clinical validation, and regulatory pathway before reaching market readiness. This can be enabled through facilitation of innovative financing models from public and private sources, support with market mechanisms to deliver affordable, easy-to-use, quality of care at the primary health level.
- Multi-Stakeholder Collaboration – Sustainable Support for Infectious Diseases
Funding for infectious diseases continues to be largely dependent on government sector in India and there is an increased need to change this paradigm. Stronger partnerships within like-minded partners (public, private and multilateral foundations) are the need of the hour to bring the focus on infectious diseases and amplify the support provided towards solving the infectious diseases problem in a comprehensive manner.
- Knowledge Sharing Platform
Building strong knowledge platforms to capture learnings and successes of health initiatives especially in the space of infectious diseases is of utmost importance to inform and influence the national health program. The best practices being followed and the fragmented data that is being generated in the infectious diseases space needs to be consolidated and communicated for larger impact that can shorten the learning curve for newer innovations.
- Market Access – Public and Private Health Systems
Infectious Diseases’ focussed innovations need to overcome the risk of market adoption and this needs to be supported by key stakeholder partnerships that facilitate adoption and market access of diagnostics by the central and state governments and the private sector.
Tuberculosis (TB)has been in existence for centuries and continues to be among the top 10 causes of death worldwide claiming nearly 1.5million lives annually. As per the Global TB Report 2018, India is the hub of the TB epidemic bearing 27% of the global disease burden.
- The National Strategic Plan 2017-2025 envisions elimination of TB in India by 2025, five years ahead of the global target to end TB by 2030
- However, achieving this goal calls for identifying and supporting innovations that can accelerate India’s fight to end tuberculosis in a non-linear and sustainable way. During the plan period, targets for TB are i) 80% reduction in TB incidence (i.e., reduction from 211 per lakh to 43 per lakh) ii) 90% reduction in TB mortality (i.e., reduction from 32 per lakh to 3 per lakh) and iii) 0% patient having catastrophic expenditure due to TB.
- India’s current TB decline rate is however very slow and are unlikely to meet the 2030 Sustainable Development Goals (SDGs) or 2035 End TB targets at this rate. Innovative and comprehensively deployed interventions are required to accelerate the rate of decline of TB incidence, to more than 10-15% annually set out by the National TB Control programme.
In alignment with the national efforts, India Health Fund (IHF) has been promoting innovations which potentially address the present gaps and challenges in the TB program. It aims to bring together resources to engender transformative change by supporting innovation that will effectively address the rapidly transforming and complex landscape of TB.
9. Annexure