
Background
India Health Fund (IHF), in collaboration with ACT Grants, IPE Global, and IKP Knowledge Park, is calling for proposals for innovative solutions, including digital health technologies, medical devices, and diagnostic tools, to address critical gaps in primary healthcare and tuberculosis (TB) care, particularly in underserved communities in India. Despite increased investments and expanded treatment coverage, TB remains a major public health challenge, with India accounting for 2.7 million cases and over 300,000 deaths in 2023. Diagnosis, treatment adherence, and prevention continue to face significant barriers, particularly among vulnerable populations.
Traditional diagnostic tools often lack sensitivity and specificity, leading to missed cases, while treatment adherence is hindered by stigma, socioeconomic constraints, and complex regimens. Drug-resistant TB (DR-TB) further complicates disease management, necessitating more effective solutions. Emerging concerns, such as subclinical and incipient TB, contribute to continued transmission, highlighting the need for advanced technologies in screening and diagnosis. Climate change further threatens TB care by disrupting healthcare access through extreme weather, displacement, and environmental stressors. Heat stress, for instance, can degrade diagnostic reagents and reduce the efficacy of heat-sensitive drugs, affecting treatment outcomes. Strengthening health systems with climate-resilient diagnostic tools and delivery mechanisms is crucial.
IHF seeks solutions that integrate health innovations and climate resilience into TB care. Proposals that enhance early detection, improve disease management, and ensure long-term health system resilience will be prioritized. By supporting transformative innovations, IHF aims to bridge existing gaps while future-proofing India’s health systems against emerging threats, ensuring equitable access to high-quality healthcare.
Scope of the call:
Problem Statement 1: Enhancing TB Screening and Diagnostics in High-Risk and vulnerable populations and addressing existing gaps in TB care.
Despite global efforts to control tuberculosis (TB), significant gaps persist in the timely detection of the disease, particularly among high-risk and vulnerable populations. Traditional TB diagnostic methods, such as smear microscopy, suffer from low sensitivity, especially in individuals with low bacterial loads (e.g., children, people living with HIV, and those with comorbidities). Additionally, the reliance on sputum-based diagnostics presents challenges in populations where sample collection is difficult, such as pediatrics and individuals with weakened immune systems. Additionally, access to rapid molecular diagnostics remains limited in many peripheral health centers, further delaying diagnosis and treatment initiation.
To address these gaps, there is a critical need for screening and diagnostic solutions that are rapid, accurate, affordable, scalable and relevant to the contextual needs of the population. These decentralized solutions should be accessible in resource-limited settings, including migrant communities, tribal populations, and difficult to reach populations. Innovations in non-sputum-based diagnostics or screening technologies, such as blood-based, urine-based, or breath-based tests, AI-assisted radiology imaging (including chest X-ray interpretation, ultrasound-based screening, and clinical decision support systems etc.), molecular and biosensor-based testing, and integrated drug resistance detection hold the potential to significantly enhance TB case detection, improve treatment outcomes, and reduce transmission.
Need # 1: Cost-effective innovations for easy sample collection, handling, and storage, which can minimize the risk of exposure.
Scope of Innovations:
- Innovations that improve sample collection (such as tongue swabs), handling, storage, pre-processing, verification, and/or reporting for efficient processing and minimizing sample loss ensuring accurate and reliable diagnostic outcomes. Applicants are encouraged to consider using thermostable sample storage solutions to prevent sample degradation in higher temperature regions.
- Innovations in sample processing that can enhance the concentration and quality of samples for more accurate testing. Additionally, incorporating heat-resistant polymers in collection and processing tools could help maintain sample viability in extreme climatic conditions.
- Innovations in sample collection tools suitable for the needs of paucibacillary TB cases such as pediatrics, people with HIV, individuals with co-morbidities, or those with low bacterial loads. Utilization of nanomaterial-based protective coating layers enhances the stability of diagnostic components may be explored.
Need #2: Accurate, rapid, affordable, and decentralized TB screening and diagnostic solutions—including non-sputum-based, point-of-care, minimally/noninvasive tools—to improve screening and detection of all forms of TB (pulmonary, extrapulmonary, drug-resistant) suited to address the needs in high-risk (migrant workers, tribals, persons with co-morbidities etc.) and vulnerable populations (pediatrics, immuno-compromised, other vulnerable populations etc).
Scope of Innovations:
- Molecular or immunoassay-based solutions that allow for decentralized testing in field settings, suitable for use by health workers in mobile clinics/limited resource settings. Integration of both drug resistance detection and TB detection into a single platform would be an advantage along with differentiation of TB and Non-Tuberculous Mycobacteria (NTM). Solutions may consider incorporating heat-stable reagents and thermostable sample storage solutions to ensure functionality in high-temperature regions.
- Effective TB triaging solutions that can aid in active case-finding, such as breath-based, sound-based, biomarker-based solutions etc. Optimized reagent formulations with heat-resistant enzymes, antibodies, and primers may be explored to ensure assay stability in extreme conditions.
- Tools that use alternate sample types such as blood or urine samples to detect low bacterial loads /paucibacillary TB cases. Integrating preservative formulations to maintain sample integrity under heat stress may be explored.
- Portable TB screening/ diagnostic devices that can integrate AI for automated image reading (e.g., chest X-rays- CAD, POCUS), reducing the need for specialists on-site. Applicants may explore using heat-resilient materials in test components to aid in increasing durability in field conditions.
- Low-cost genomic sequencing, (whole genome/targeted genome sequencing (WGS/tNGS) based devices), to detect DR TB and aid in transmission dynamics. Encapsulation techniques using heat-resistant coatings may be explored to enhance reagent longevity.
Need # 3: Scalable and Replicable ready to deploy models for integrated TB screening and diagnostics.
Scope of Innovations:
- Scalable, cost-effective approaches that maximize TB case detection within the existing TB care continuum. These models should leverage public health infrastructure, minimize additional human resource requirements, and integrate seamlessly into the ongoing program. Implementation may involve combining two or more complementary technologies (e.g., molecular, immunoassay, AI-driven imaging) to create an end-to-end, patient-centric, and geographically adaptable screening and diagnostic workflow. Applicants are encouraged to consider climate-resilient designs and approaches to ensure the program’s effectiveness in diverse settings, for e.g. the use of packaging innovations with thermal insulation to protect test components from heat exposure to navigate climate induced supply chain disruptions.
- Innovative deployment models that integrate TB diagnostics with screening for co-infections/co-morbidities (HIV, diabetes) and other respiratory diseases to provide a holistic lung health assessment. By combining TB detection with broader disease surveillance, these approaches can improve overall health outcomes while enhancing program efficiency and cost-effectiveness. Integration of phase-change materials in test transport and storage solutions to ensure reagent viability in resource-limited, high-temperature settings may be considered.
Additionally, applicants may also consider designing a program incorporating a combination of the above-mentioned approaches.
Problem Statement 2: Bridging the Gaps in TB Detection Across the Disease Spectrum to address TB’s silent and emerging forms.
Traditional TB classification, which categorizes the disease as either latent TB infection (LTBI) or active TB, no longer fully captures the complexity of TB progression. Recent studies have demonstrated that TB exists on a dynamic spectrum, ranging from latent, incipient to subclinical TB, where individuals harbor Mycobacterium tuberculosis (M. tuberculosis) but remain asymptomatic while still capable of transmitting the infection. Research indicates that TB transmission occurs from individuals with subclinical TB before they progress to symptomatic active disease. Furthermore, a significant proportion of individuals with LTBI—estimated at 5-10%—will progress to active TB in their lifetime, with higher risks among immunocompromised individuals and household contacts of active TB patients.
To effectively reduce TB incidence and improve case detection, diagnostic strategies must evolve beyond conventional methods to detect TB across this spectrum. Advancements in non-invasive biomarker-based assays (e.g., host RNA signatures, TB antigen-based skin tests etc.), AI-powered CXR and CAD algorithms, and next-generation molecular diagnostics (e.g., CRISPR-based, multi-target NAATs etc.) offer the potential to detect TB in its earliest forms. Additionally, innovative LTBI diagnostics that may also differentiate incipient TB from persistent LTBI and predict progression to active TB are critical for targeting preventive therapy to individuals at the highest risk, including healthcare workers and close contacts of active TB patients. Integrating these advanced diagnostic tools into routine TB programs can bridge detection gaps, reduce transmission, and accelerate TB elimination efforts.
Need #4: Tools for detecting subclinical/incipient in individuals with sporadic/no symptoms, particularly in high-burden regions.
- Performance enhancements (such as improvements in accuracy, sensitivity, specificity, limit of detection etc.) or repurposing existing tools that can be suitable for the detection of sub clinical TB.
- Biomarker based innovations that demonstrate potential to identify TB in asymptomatic individuals towards enabling early intervention in populations at high risk.
Need #5: Rapid diagnostics for latent tuberculosis infection (LTBI) in high-risk populations
Scope of Innovations:
- Non-invasive diagnostic tools that can be used for LTBI screening such as saliva-based tests, reducing the barriers to regular screening and early intervention.
- Innovations in multi-antigen assays or immunoassays to detect latent TB in high-risk populations such as healthcare workers and TB contacts. The ability to differentiate LTBI from active TB or predict progression outcomes may be an added advantage.
- Rapid, cost-effective solutions for LTBI that has high specificity, shorter Turnaround Time (TAT) reducing the burden of false positives from BCG vaccination aimed at improving the uptake of TB Preventive Therapy (TPT).
Problem Statement 3: Digital Innovations for Strengthening TB Screening, Diagnosis, and Patient Management.
Traditional TB diagnostic methods often face challenges related to accessibility, turnaround time, and reliance on specialized skillset. These limitations hinder timely diagnosis and treatment initiation, particularly in low-resource and high-burden settings. Leveraging AI, machine learning, and integrated digital platforms that not only address TB but also other lung health conditions, hold the potential to improve diagnostic accuracy, streamline patient tracking, enhance treatment adherence, and provide lung health assessment. Digital platforms could also integrate diagnostic results with patient management systems while reducing errors and inefficiencies arising because of dependency on manual processes. While innovations such as AI-driven diagnostics, Digital Adherence Technologies (DATs), and automated reporting tools (e.g., QR-code-based sample tracking) demonstrate promise, their isolated application fails to address systemic inefficiencies in TB care pathways. This highlights the need for multi-component, context-sensitive designs that have the potential to strengthen the existing system.
Need #6: Digital health tools to improve efficiency, monitoring, and reporting of TB diagnosis, treatment, and follow-up and other associated lung health conditions.
Scope of Innovations:
- Comprehensive lung health platforms/panels to differentiate between TB and other common respiratory infections such as pneumonia, COPD exacerbations, bacterial and viral lower respiratory tract infections and other overlapping lung health conditions such as silicosis, pneumoconiosis, bronchiectasis asbestosis etc. ).
- Integrated digital health solutions that combine diagnostic data with patient management systems for improved tracking, adherence, and follow-up care, while enabling real-time reporting and monitoring of TB cases and treatment outcomes.
- AI-based systems to minimize errors due to subjectivity errors associated with the use of existing tools, solutions that aid in automating data entry, and provide actionable insights to support decision making.
- Affordable offline-enabled systems to track TB treatment adherence in populations without internet access or smartphones.
Program Offerings
- Milestone-based funding support.
- Need-based mentoring/knowledge building by a panel of experts for guidance on study design and validation, regulatory landscape know-how, market access support.
- Deployment support for ready-to-implement technology/group of technologies in an integrated approach, subject to the innovator having required permissions from the concerned agencies.
- The innovation should be TRL-4 and above (See FAQ for an explanation of TRL)
-
• Promising proposals at TRL 4, 5, 6 & 7 will be supported for product development, clinical validation, regulatory approvals, and multicentric trials.
• Promising innovations at TRL 8 & 9 will be supported for evidence demonstration, community validation, and acceleration. - Access to resources for adoption in the public health system, including support towards enabling demonstration through pilots and advisory on go-to-market strategy.
- Access to health economics research and outcome evaluation opportunities to assess the cost-effectiveness of the innovation and guide its development and implementation.
Project Duration and Budget
The project duration along with clear project outcomes defined by the applicant will be subject to detailed evaluation and approval by the funding committee. Promising proposals will be supported with funding by IHF, ACT Grants, IPE Global and IKP Knowledge Park contingent on milestones achieved at different stages.
EVALUATION & SELECTION CRITERIA
- Proposals should demonstrate a clear understanding of the primary healthcare challenges being addressed and the potential impact on healthcare delivery and patient outcomes. They should also outline a clear path to implementation, including any necessary partnerships or collaborations. We are open to both digital and digitally enabled technologies.
- We welcome proposals from individuals, startups, academic institutions, and established companies. Proposals will be evaluated based on their innovation, feasibility, potential impact, and scalability
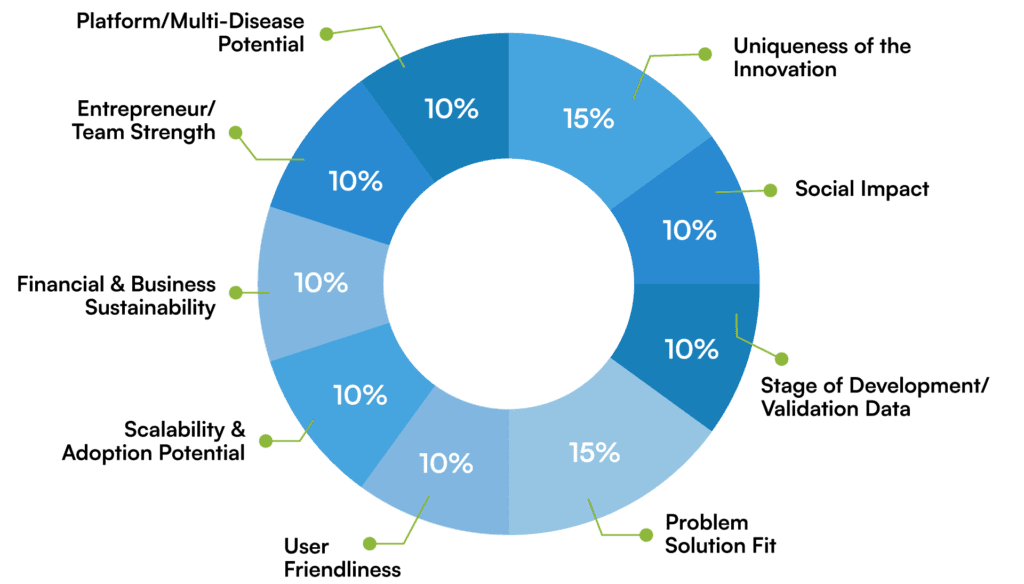
The applications will be evaluated on the following parameters:
Apply Here
- Applications must be submitted by entities registered and incorporated in India.
- The proposal is relevant and aligned to at least one of the problem statements given above.
- Transparency in furnishing relevant data, including validation/pilot data, in support of the proposed innovation, should be agreeable to the applicant.
- The innovation should be demonstrating improvement over the currently available solutions or approaches in terms of sensitivity, specificity, accuracy, safety, turnaround time, usability in peripheral settings, unit pricing.
- Innovation should have the potential for scale-up across diverse settings.
- It is desirable if the innovation is amenable to being multi-disease applicable.
- As suggested through this document, applicants are encouraged to consider the impact of climate change as a key factor for design, development and deployment of their innovations.

The following falls outside the scope of the Call and will not be supported by partners:
- Basic science research.
- Epidemiological studies/surveys/disease burden analysis.
- Proposals focused on pure service delivery.
- Innovations in the ideation/ proof-of-concept stage/ formative studies.
- Applications which propose incremental solutions without a clear innovative element.
- Nonalignment with national goals of Government.
- Nonalignment with the Sustainability Development Goals (SDGs) related to healthcare.
Timeline and Process Flow for Applications:
Process step | Date(s) | |
Announcement for Applications | 24th March 2025 | |
| ||
Application submission deadline | 30th April 2025 (5 weeks) | |
| 12th May 2025 | |
| 19th May 2025 | |
| Before 31st May 2025 (Subject to experts’ availability) | |
Internal due diligence, including site visits and approval process | June- Mid July | |
Due diligence at operational and technical levels and third-party financial audit of the awardee will be conducted by organizers of the call. Satisfactory completion of the same is mandatory for the issuance of award letter. | ||
Decision on awardees and intimation of the same (based on only those applications received by the 5th of May 2025 | Sept 1st week 2025 (Subject to the satisfactory completion of due diligence & approval process) | |
* Note: Any changes in timelines will be intimated to relevant applicants at every stage.
For queries, please reach out at contact@indiahealthfund.org

ABOUT INDIA HEALTH FUND
India Health Fund (IHF), incorporated as Confluence for Health Action and Transformation Foundation (under Section 8 of Companies Act, 2013), was seeded in 2017 by Tata Trusts and with strategic support from The Global Fund. IHF was conceived to accelerate India’s progress towards the elimination of infectious diseases. IHF does this by addressing the gaps in funding for product development, in mentorship and in market access that are often faced by small and mid-size entities with powerful ideas that have the potential to significantly improve outcomes in the prevention, diagnosis, and treatment of infectious diseases. IHF also works to develop effective business models, implementation partnerships and financing mechanisms which help to significantly scale up these solutions – enabling impact at scale.
ABOUT ACT GRANTs
ACT is a non-profit venture philanthropy platform that was set up in 2020 in response to the COVID-19 crisis and is built upon the premise that an entrepreneurial mindset, technology & innovation and collective action have the power to create meaningful social impact at scale. The organization is driven by a bias for action to catalyze social change through collaboration across the board – VCs, startup founders, donors, NGO’s, expert advisors, government stakeholders as well as the public at large. ACT For Health aims to improve access to quality and affordable healthcare in the areas of primary care, diabetes, cancer, tuberculosis and mental health by supporting digital/MedTech/deep tech innovations in these spaces.


About IPE Global
India Health Fund (IHF), incorporated as Confluence for Health Action and Transformation Foundation (under Section 8 of Companies Act, 2013), was seeded in 2017 by Tata Trusts and with strategic support from The Global Fund. (IHF) was conceived to accelerate India’s progress towards the elimination of infectious diseases. IHF does this by addressing the gaps in funding for product development, in mentorship and in market access that are often faced by small and mid-size entities with powerful ideas that have the potential to significantly improve outcomes in the prevention, diagnosis, and treatment of infectious diseases. IHF also works to develop effective business models, implementation partnerships and financing mechanisms which help to significantly scale up these solutions – enabling impact at scale.
About IKP Knowledge Park

Co-Funding for Proposals
IHF, ACT Grants, IPE Global and IKP Knowledge Park are strong believers in collaborative funding models to bring about greater impact, multiplier effect, and demonstrate innovative models of financing in the infectious disease ecosystem. This RFP will be co-funded by IHF, ACT Grants, IPE Global and IKP Knowledge Park. While IHF and ACT Grants and IKP Knowledge Park largely derisk through non-dilutive funding (non-returnable grants models), IPE Global also enables others forms of capital like returnable grants, debt , guarantees etc. The aim of the collaboration is to develop a larger pool of funds to support more innovations, enable suitable forms of capital depending on the stage of innovation (mid to late stage, deployment project etc.) with an endeavor to develop sustainable models. The scope of this call for proposals involves assessing the mid-to-late stage ready to deploy innovations for funding and other forms of support as listed in the programs offering section.
As an important part of the evaluation process, we request all applicants to keep IHF, ACT Grants, IPE Global and IKP Knowledge Park teams updated on any co-funding and partnership efforts with reference to the application to be submitted in this call. We value transparency in the funding process.
Disclaimer: The call is a voluntaryand discretionary measure in addressing the public health problem of infectious diseases in India. Therefore, IHF, ACT Grants, IPE Global and IKP Knowledge Park reserve the right to the following:
- Disqualify proposals that do not fall within the focus areas stated below, do not meet the eligibility criteria laid out below or those which plagiarize the works of others.
- Modify and refine proposals before final selection.
- Modify budgets based on rationale and justification.
- Not provide or justify reasons or feedback on rejection of proposals.
- Verify any information provided by application through different sources.
- Nullify this call at any time owing to any reason.
FREQUENTLY ASKED QUESTIONS (FAQs)
Can I submit more than one application for different innovations?
Yes, you can submit multiple applications if they fall within the above focus areas and evaluation criteria. Please note that each application is associated with one email address. Therefore, please use different email addresses if you are making more than one application.
How many projects will be selected for support per problem statement?
We do not have any limit on the number of awardees per problem statement. The final funding decision on proposals will be purely assessed on merit.
What would be the funding size for my innovation?
India Health Fund and partners do not suggest any funding (grant and/or equity) size for the proposals submitted through the RFP. The financial ask should be realistic and in alignment with the proposed work. The decision to approve the funding request, periodicity, and the conditions of disbursements lie solely with IHF and partners.
Who will provide funding for my innovation?
IHF and partners are strong believers of collaborative funding models to bring about bigger impact and a multiplier effect in the infectious disease ecosystem. The proportion of co-funding and support sought from IHF, and partners will be assessed and may vary on a case-to-case basis. As an important part of the evaluation process, we request all applicants to keep IHF and partners updated on any existing co-funding and partnership efforts regarding the application. We value transparency in the funding process.
For what purpose can the funding be utilized?
This request for proposal is meant for innovations at or above TRL-4. Therefore, funding can be utilized for product/technology development, clinical testing and validation, commercial validation, and associated expenses for the same (e.g. labour and talent, consumables, equipment, project-related travel, etc.). The funding should solely be used for restricted work within the focus areas mentioned.
What does due diligence entail?
Due diligence includes a holistic evaluation of your organization and application. It will cover aspects including, but not limited to, technical and operational feasibility and rationality, the progress of clinical study, financial assessment, and refinement of the business model. It will also include reflecting on the long-term vision, goal, and intermediary outcomes envisaged in the proposal, project implementation plan, and competence of the applicant. Due diligence may require multiple iterations between IHF & partners, technical experts, external independent auditors, who may be hired to assist in the process, and applicants. Awards will be announced purely on merit basis and after completion of a rigorous due diligence process. Mere satisfactory completion of due diligence does not entitle an applicant to funding and support.
Can a soon-to-be-registered organization apply?
No. Once shortlisted, applicant organizations will have to produce relevant supporting documents to support their registration status, without which the application will not move through the selection process.
What are the various levels of TRL?
TRL 9 – Technology has been applied in its final form and is operational.
TRL 8 – Technology is proven and developed but not yet operational or applied anywhere.
TRL 7 – Actual system prototype is near completion or ready and has been demonstrated in an operational environment or is at pilot level.
TRL 6 – The prototype is being tested in a simulated operational environment or a high-fidelity laboratory environment.
TRL 5 – Technology has been put together and can be tested in a simulated environment.
TRL 4 – Basic technological components have been integrated to establish that they work together.
TRL 3 – Proof-of-Concept stage / Active R&D/ Advanced prototype has been initiated. This includes analytical studies and laboratory studies to physically validate the analytical predictions of separate elements of the technology.
TRL 2 – Technology concept/application formulated.
TRL 1 – There are research articles to support the technology’s basic properties.
