
Early detection and diagnosis of infectious diseases is a necessity to reduce the rate of infection.
Infectious diseases tend to spread rapidly after a short incubation period. Therefore, it becomes imperative to detect the diseases early and put adequate treatment and containment measures in place. This can help avoid more than 14 million deaths worldwide, every year. In order to accurately assess the quality of the tests, World Health Organization, in early 2000, came up with the ASSURED characteristics- Affordable, Sensitive, Specific, User-friendly, Rapid, Equipment-free, and Delivered to those in need. This has now been updated to being called REASSURED- to include Real-time connectivity and Ease of Specimen collection and account for the digitization of the healthcare diagnostics industry. Much of the traditional diagnostic methods were focused on microscopy and culture staining which were time consuming and lacked accuracy. Conducting these tests also required highly trained staff and careful sample collection. Despite these challenges, culture-based methods remain the gold standard for clinical diagnosis. For example, antibiotic susceptibility testing methods are still used to a great extent to identify antibiotic resistance.
To solve some of these challenges associated with culture-based methods, point of care diagnostic tests were developed focused on antigen-antibody interactions- enzyme-linked immunosorbent assays, optical immunoassays, and lateral flow assays (LFAs) based on immunochromatography. These tests are available for a range of respiratory tract infections, diarrhea, and some fungal tests as well. However, stability, manufacturing strength, operational standards, and potential for technical errors due to challenges in administering the tests have restricted their use as screening devices with a need for a more sophisticated and accurate test recommended for diagnosis.
Molecular diagnostics tests based on Polymerase Chain Reaction (PCR) or other isothermal nucleic acid amplification tests (NAAT) are quickly becoming the standard due to the significant advantages they offer with respect to faster turnaround time (TAT), better sensitivities and specificities with the ability to amplify a smaller signal, lower costs and simplicity of operating the system. They also have the ability to detect the infection at a very early stage, sometimes even at DNA copy numbers between 10-100. With the rise in antimicrobial resistance, molecular diagnostic solutions also have the ability to identify the different strains and design solutions catered to detect very specific markers against individual microbial strains.

Why are molecular diagnostics tools being used now?
The new currency in the genomics era that we live in is DNA. Thanks to sophisticated “Next Generation Sequencing” (NGS) technologies, the price of sequencing a full human genome has decreased from $10,000 in 2011 to less than $1000 now. Sequencing has become more accessible as costs have decreased. NGS machines were installed in many new labs during COVID-19 which has provided a boost to the industry. Large-scale and rapid creation of genomic data has been made possible by reduced costs, increased throughput, and simpler access. The discovery of novel biomarkers for a variety of malignancies and infectious diseases has fueled the expansion of the molecular diagnostics sector.
Point of care (POC) molecular diagnostics will require breakthroughs in amplification technologies.
PCR-based diagnostic assays have several challenges. To begin with, they need costly machinery, knowledgeable specialists, and a variety of reagents for the various stages. In order to amplify the target DNA, they also need a lot of cycles that alternate between hot and low temperatures. Because of these shortcomings, PCR testing are now only conducted in central labs with adequate equipment and lengthy turnaround times. Therefore, there is a need for PCR test alternatives that may be completed at the point of care (POC).
Research labs worldwide have been working on several substitutes for PCR-based diagnostics in an effort to address some of the limitations of PCR testing. The capacity to amplify nucleic acids is important to each of these methods: DNA and RNA. A few of the methods created include rolling circle amplification (RCA), strand-displacement amplification (SDA), loop-mediated isothermal amplification (LAMP), nucleic acid sequence-based amplification (NASBA), and recombinase polymerase amplification (RPA). Their main benefit over PCR is that they are isothermal procedures, meaning they operate at a steady temperature. They thus make excellent candidates for easily administered tests that may be completed at the POC and at a reasonable cost. They do, however, have difficulties matching the PCR tests’ high sensitivity and specificity, which call for ingenious engineering and study. Additionally, distinct primer design is necessary to create these isothermal tests for various illnesses and creating primers for these substitutes is typically a more challenging undertaking than creating primers for a PCR.

Startups are also looking at improving the existing PCR technologies by developing portable PCR machines or PCR on a chip or microfluidic devices. Significant research is also being done to increase the sensitivities of the assays by using nanotechnology-based approaches.
Investments into molecular diagnostics saw a rise during COVID-19.
Although molecular diagnostics tools have been around for more than 30 years, investments into startups in this space have seen a steady rise since 2013, driven by the advancements in sequencing technologies and gene editing tools such as CRISPR, as well as new isothermal molecular diagnostic technologies. While the initial focus for these startups was on cancer diagnostics, COVID-19 gave a major push towards early detection of infectious diseases. RT-PCR and other isothermal tests such as Loop Mediated Isothermal Amplification (LAMP) became a standard for screening and diagnosis during 2020-21. During 2021, molecular diagnostic investments, including debt, follow-on public offerings (FOPOs), initial public offerings (IPOs), private investments in public equity (PIPEs) and venture capital (VC) reached a total of $8.8 billion, up from $7.6 billion in 2020. While a huge percentage of the investments were still focused on cancer diagnostics, startups focused on early detection of infectious diseases have also seen a rise in funding.
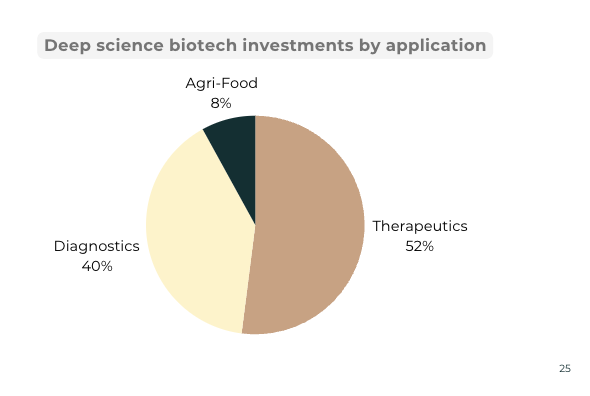
Source: India Deep Science Tech Report
In India, diagnostics received 40% of the total deep science biotech investments over the last decade. MolBio Diagnostics raised the highest amount of funding with a total of $117.5 M. The company has developed the Truenat portable PCR solution for Malaria, MTB, Dengue, Chikungunya and many other infectious diseases. The company was able to expand and install its devices extensively during the COVID-19 pandemic and cater to the need for rapid diagnosis of the COVID-19 strains. Medgenome, which has raised more than $200 million in funding, also offers diagnostics kits for tuberculosis, gastrointestinal and COVID-19 infections. Startups such as Mylab Discovery Solutions, NeoDx have also developed kits for COVID-19 detection along with molecular diagnostics solutions for TB detection. New generation companies such as D-Nome are using advances in synthetic biology and protein engineering to develop room temperature PCR solutions for point of care diagnostics.
These startups are developing highly innovative products which are globally competitive and are solving globally relevant problems related to the diagnosis of infectious diseases. Navigating the challenges in scaling up these solutions will be key over the next few years and investments will be focused on achieving regulatory clearances, intellectual property through patents, clinical trials and any other strategies surrounding product commercialization. Point of care tests with high sensitivities and specificities for early diagnosis of infectious diseases are the need of the hour and innovations in the PCR tools to detect multiple diseases at once (multiplexing) as well as non-PCR based isothermal amplification tests will play an important role going forward.
About the Author:

Suraj Nair is part of the deep science technology investments team at Ankur Capital. He leads the investments into disruptive solutions in biotechnology for healthcare and climate-related applications. Suraj has a Masters degree in bioprocessing/biotechnology from ICT Mumbai.
